
Around 210 BC, in the company of several hundred virgins, court
alchemist Xu Fu was dispatched by the Qin Dynasty emperor, Qin Shi
Huang, to find The Elixir of Immortality. He was never heard from again.
Taking matters into his own hands, the emperor instructed his court
alchemists to devise their own potions for eternal youth.

|
| Emperor Qin Shi Huang, the first emperor of a united China and builder of the Great Wall, longed for The Elixir of Immortality. Despite the work he spearheaded to consolidate Chinese characters into writing, there remain more than 100 characters that convey the notion of longevity. Advertisement
|
Around 210 BC, in the company of several hundred virgins, court alchemist Xu Fu was dispatched by the Qin Dynasty emperor, Qin Shi Huang, to find The Elixir of Immortality. He was never heard from again. Taking matters into his own hands, the emperor instructed his court alchemists to devise their own potions for eternal youth. Although their efforts proved less than successful, killing Qin Shi Huang with a lethal concoction of mercury, the era marked a period of intense Chinese interest in means to advance longevity. The classic Chinese alchemical book, the Tan Chin Yao Ch’eh (Great Secrets of Alchemy) dating from around AD 650, talks about the creation of potions for immortality, tonics for a variety of diseases and the fabrication of precious stones. Many of these formulations featured substances such as mercury, mercury salts, sulphur and arsenic, which, far from contributing to longevity, are actively toxic and were implicated in the poisoning deaths of several emperors. Chinese interest in alchemy eventually waned with the rise of Buddhism, which purported alternative, and less lethal, paths to immortality.
Western alchemists picked up the quest for immortality with efforts to uncover the philosopher’s stone – a substance which, apart from converting base metals to gold, was supposed to confer the ability to defy aging on those who consumed it. The 15th century French scrivener, Nicolas Flammel, developed a posthumous reputation for his alleged discovery of the Elixir of Immortality and the enigmatic Louis XV courtier, the Comte de Saint-Germain, was also said to have discovered the means to agelessness. Confirmed accounts of his death, however, limited somewhat the credibility of the story.
Tales of fabled waters with special restorative powers were commonplace throughout history and date from at least as early as the fifth century BCE when the Greek historian, Herodotus, described a fountain of longevity-enhancing water located in the land of the Ethiopians. Narratives of this kind were particularly widespread in the 16th century, when the legend became attached to the Spanish explorer Juan Ponce de León, first governor of Puerto Rico. According to a fanciful account that features a combination of New World and Eurasian elements, Ponce de León was searching for the magical waters when he discovered what is now Florida. Although there is no evidence he spent any time looking for the site of the vitality-restoring water, Ponce de León’s name has since become synonymous with the Fountain of Youth.
REDISCOVERING THE FOUNTAIN OF YOUTH
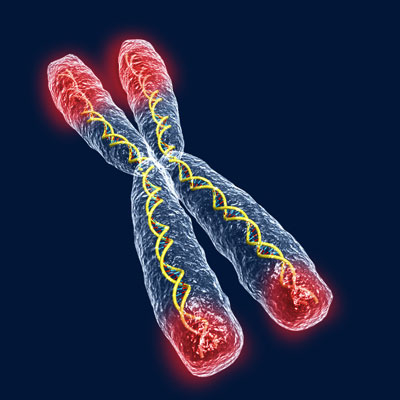 |
| Telomeres consist of thousands of repetitions of the DNA nucleotides TTAGGG at the ends of chromosomes. |
The very human fascination with eternal youth and immortality likely began the moment after we became aware of our own mortality. Until the 1990s, the search for means of life or youth extension had largely gone into abeyance because of a general acceptance that aging, and the diseases and infirmities that come with it, are simply a part of the normal life cycle of our own limited existence. Aging, our intuition tells us, is not a disease but a natural process – the progressive buildup of defects that our bodies simply lack the ability to repair. Consequently, questions of how we age, how long we’ll last, and what we can do about it were not subject to serious research. What has changed in recent years is the growing consensus that at least some of the diseases associated with old age – like osteoporosis, heart disease, diabetes and cancer are not only manageable but potentially curable. Maybe we can promote life extension by eliminating the chief causes of death that come between us and our biblical, three-score and 10 or more years. And if we can control the main causes of age-related death, maybe we can also manipulate the basic building blocks of our bodies to extend life span itself.
Surging interest has been propelled by a redefinition of aging as an underlying timing mechanism for all chronic diseases; the accumulated risks for acquiring the diseases that eventually end the lives of most of us. This has led to a paradigm shift in how we view disease, aging and longevity. As often happens at times of transformative change, it starts with a simple change in perspective. What was until recently seen as an inescapable path to decrepitude and death now offers new hope. Suddenly there is an explosion of renewed interest in the ancient quest for disease-free, life extension – perhaps immortality itself.
WHY DO WE AGE?
THE TROUBLE WITH TELOMERES
Theories of aging fall into two groups. One group takes a “planned obsolescence view” whereby aging follows a predetermined biological timetable. These programmed theories hold that senescence, the progressive deterioration of bodily functions over time, may be an extension of the same general processes that control childhood growth and development. Damage or error theories, on the other hand, accentuate the cumulative environmental assaults that our bodies endure over time. Theories from both these camps are not necessarily mutually exclusive and the processes they describe may operate in tandem to cause progressive systemic deterioration, open the pathways to disease and lead to our eventual death.1
Programmed theories include programmed longevity wherein aging results from the sequential switching on and off of certain genes; the endocrine theory wherein biological clocks act through hormones to control the pace of aging; and the immunological theory, a predetermined decline in immune system function leading to an increased vulnerability to infectious disease, and so to aging and death.2
In recent years, the speculative but tantalizing role of telomeres in aging has made it the focus of almost science-fictional fascination. Telomeres consist of thousands of repetitions of the DNA nucleotides TTAGGG at the ends of chromosomes. The repetitive structure stabilizes and protects our chromosomes, forming a tight bond between the two strands of the DNA. These chains of nucleotides prevent chromosome ends from being mistaken for broken pieces of DNA but become shorter with each cell division. Every time a cell divides it loses up to 200 DNA base pairs off the telomere ends. When telomeres get short enough, after about 100 divisions, our cells cannot continue dividing and eventually die.3 Like a metronome clocking the tempo of a cell’s senescence, this mechanism for tracking a cell’s age has led to speculation that telomeres may also play an active role in regulating cellular life span.4 According to the telomere theory of aging – one component of the programmed theories – the progressive erosion of these chromosome ends is a major factor in the processes of senescence leading ultimately to our death. Stop that process and, just maybe, immortality is within reach.
Indeed, telomere length and life span do appear to be associated. A landmark study in The Lancet5 “found that otherwise normal people over 60 who started out the study with short telomeres were more likely to die over the next 17 years than those with long telomeres. And shortened telomeres do appear to correlate with a higher risk of certain diseases, sometimes quite strongly. Numerous studies have found a connection between short telomeres and higher risk of cardiovascular disease, and in the Lancet study, those with short telomeres were three times as likely to die of heart disease and more than eight times more likely to die of infectious disease than those with longer telomeres.”6
Telomeres’ accomplice in aging is the enzyme telomerase. Telomerase, sometimes referred to as an immortalizing enzyme, protects and stabilizes telomeres “similar to plastic tips on shoelaces.”7 It maintains telomeres in our reproductive and stem cells but not in the rest of the body and is part of a compensatory system for the cellular shortening of telomeres.8 In a recent break-through study, it was found that, as well as helping keep telomeres from deteriorating, it also reverses the aging of telomerase-deficient rats. In a transformation the lead researcher described as “akin to a Ponce de León effect,” lies the first compelling evidence of aging reversal in a high-level organism.9
A FAUSTIAN BARGAIN
So far, so good. Like all “pact with the devil” stories, however, the neat relationship between telomeres and their companion lengthening enzyme comes with a catch. In Christopher Marlowe’s Renaissance play, The Tragicall History of the Life and Death of Doctor Faustus, Faustus deeds his soul to Lucifer in exchange for limitless knowledge. The deal includes the services of the sub-devil Mephistophilis, nominally Faustus’s servant but in reality his master. In time, Faustus comes to appreciate that the deal fell short of his expectations and that knowledge without wisdom is mortally dangerous.
The devil’s fine print here is in the unsettling relationship between telomerase and cancer. Theoretically, boosting telomerase activity would continuously renew our telomeres and thereby slow aging or stop it altogether. However, activation of telomerase appears to be responsible for the sustained growth and malignancy of many tumours. It also confers immortality on cancer cells. “In immortal cancer cells, telomeres act abnormally – they no longer shrink with each cell division,” enabling them to replace lost sequences and divide indefinitely.10 Although telomerase is not present in most cells, it gets reactivated in cancer cells, so telomerase-enhancing treatments aimed at slowing aging might also increase the risk of cancer.
“As with all of our other genes, the DNA that encodes the telomerase enzyme is present in all of our cells – but because it’s needed only after quite a few cell divisions have occurred, it’s not needed in most cells for most or all of the time, so it’s turned off. This widespread lack of the need for telomerase is used by evolution as a key component of our defence against cancer, because having a limit to the size and renewal of telomeres prevents our cells from replicating themselves indefinitely – the crucial hallmark of cancer.”11 Studies in mice have shown that elevated telomerase activity leaves the animals more susceptible to skin tumours and breast cancer. Conversely, blocking the enzyme causes “any and all the aspiring cancers we developed to fizzle out before they became life-threatening indeed, before many of them even became actual cancers.”12
So we are faced with a Faustian bargain: enhance telomerase activity and slow aging but potentially heighten our odds of developing cancer. Reduce or eliminate telomerase altogether and maybe do away with cancer as a killer among humans but at the cost of accelerating our decline with age and possibly cutting short our own modest life span. The deeply interwoven relationship between telomeres and telomerase in aging and cancer promises both will remain the subject of near hypnotic fascination for years to come.
BEYOND IMMORTALITY
Telomere-centred research has invited far-reaching speculation around the enticing possibility that, by halting the processes of senescence, we may one day be able to engineer indefinite human life spans with no biologically fixed termini. The role of telomeres in aging and health, however, also has implications across a number of disciplines, not least of which, is chiropractic. At the 2011 Canadian Memorial Chiropractic College Research Symposium, for example, Dr. Aviad Haramati of Georgetown University looked at the place of mind-body medicine in the training of health professionals. His research references factors such as accelerated telomere shortening in response to life stress, perceived stress and telomere length, and possible pathways by which chronic stress impacts telomeres. Research of this kind raises provocative questions about the nature and timing of health-care provider interventions as well as the role of stress management, lifestyle balance and other aspects of psychological well-being in health professional education. We will look at some of these questions in the context of quality-of-life issues later in our exploration of aging and longevity.
In Part 2, we will examine other factors in the aging and longevity discussion, strategies to promote longevity and genetic determinants of life span.
REFERENCES
- National Institute on Aging, National Institutes of Health (US). Aging under the microscope: a biological quest. 2006. NIH Pub. No. 02-2756: 6.
- Ibid.
- Shay, JW. Aging and cancer: are telomeres and telomerase the connection? Molecular Medicine Today, Reviews. 1995 Nov; 1(8):379.
- National Institute on Aging, National Institutes of Health (US). Aging under the microscope: a biological quest. 2006. NIH Pub. No. 02-2756: 16.
- Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003 Feb 1; 361(9355):393-5.
- Greenwood, V. What will our telomeres tell us? Discover Magazine. 2011 May 18. Available from: http://discovermagazine.com/2011/may/18-what-will-our-telomeres-tell-us
- Shay, JW. Aging and cancer: are telomeres and telomerase the connection? Molecular Medicine Today, Reviews. 1995 Nov; 1(8):379.
- Katrin, Susan. Can a pill keep your DNA young? Discover Magazine. 2010 June 11. Available from: http://discovermagazine.com/2010/may/20-can-a-pill-keep-your-dna-young
- Keim, B. Telomere tweaks reverse aging in mice. Wired Science. 2010 Nov 29. Available from: http://www.wired.com/wiredscience/2010/11/mouse-aging-reversal/
- National Institute on Aging, National Institutes of Health (US). Aging under the microscope: a biological quest. 2006. NIH Pub. No. 02-2756: 16.
- De Grey, A, Michael R. A modest proposal: how to stop aging entirely. Discover Magazine. 2009 September 23.
- Ibid.
Steve Zoltai is the collections development librarian and archivist for CMCC and is a member of the Canadian Chiropractic Historical Association. He was previously the assistant executive director of the Health Sciences Information Consortium of Toronto. He has worked for several public and private libraries and with the University of Toronto Archives. Steve comes by his interest in things historical honestly – he worked as a field archeologist for the Province of Manitoba. He can be contacted at szoltai@cmcc.ca.
Print this page